Gut Virus Linked to Alzheimer’s: New Research Highlights a Surprising Brain-Gut Connection
A newly published study in the 19 December 2024 issue of Alzheimer’s & Dementia challenges conventional wisdom about Alzheimer’s disease, revealing how a common virus—human cytomegalovirus (HCMV)—may fuel a distinct subtype of Alzheimer’s in up to 45% of cases. Led by Dr. Ben Readhead at Arizona State University and Banner Alzheimer’s Institute, this research suggests that HCMV—generally dormant in healthy individuals—can become chronically active in the gut and potentially migrate to the brain, disrupting immune processes and sparking Alzheimer’s pathology.
“We’ve identified a potential new subtype of Alzheimer’s that seems to be driven by HCMV,” says Dr. Readhead. “It underscores how crucial the gut-brain connection is and opens new avenues for both diagnosing and treating this devastating condition.”
Below is an in-depth Q&A that covers everything from the virus’s prevalence to the proposed mechanism of its path through the vagus nerve, as well as implications for future therapies and research directions.
Q: Could you elaborate on HCMV’s prevalence, typical course of infection, and health implications for both immunocompetent and immunocompromised individuals?
A: HCMV is part of the herpesvirus family and is extremely common worldwide. Many people acquire it in childhood through contact with bodily fluids. In healthy (immunocompetent) individuals, the virus usually causes mild or unnoticed symptoms, then settles into a latent (dormant) state. However, immunocompromised individuals—such as transplant recipients, the very young, or the elderly—can develop severe complications if HCMV reactivates, potentially affecting the eyes, liver, or brain (though this is relatively rare).
Our research explored how persistent viral infections might contribute to Alzheimer’s disease. HCMV was of particular interest because of its capacity to remain dormant and to occasionally reactivate in ways that may influence chronic inflammation and immune responses in the body, including the brain.
Q: How did your investigation lead to discovering the connection between HCMV and Alzheimer’s disease?
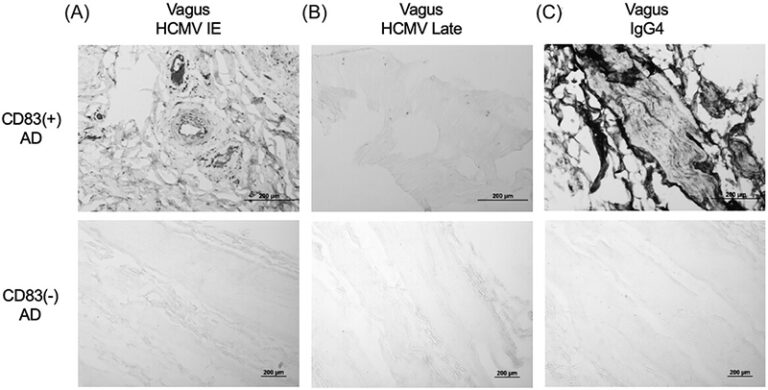
A: We first observed an unusual type of immune cell—CD83-positive microglia—in 25% to 45% of Alzheimer’s patients’ brains, suggesting a distinct form of the disease. When we analyzed the gut tissue of these patients, we found elevated levels of IgG4 antibodies, hinting at a viral or microbial trigger.
Using advanced analyses of spinal fluid, we uncovered a strong HCMV signal in patients who had these CD83-positive microglia. Further study showed HCMV in both the gut and brain tissue of these individuals, building a solid case that chronic intestinal infection by HCMV contributes to Alzheimer’s pathology in this specific subgroup.
Q: Could multiple infections or pathogens, like HCMV, work together or have a synergistic effect in Alzheimer’s disease?
A: We think multiple pathogens might sometimes interact synergistically, escalating inflammation or immune dysregulation. HCMV, however, stands out for its unique association with CD83-positive microglia and IgG4 antibodies. IgG4 is a low-inflammatory antibody subtype that only makes up about 5% of total antibodies; rather than aggressively neutralizing pathogens, it tends to bind to antigens, but won’t trigger further inflammatory events.
This distinctive anti-inflammatory response could mean one of two things:
* It’s the immune system’s way of limiting collateral damage, or
* It’s an adaptation driven by the virus to evade the immune response.
Either way, HCMV’s relationship with IgG4 seems to set it apart from other viruses, such as HSV-1.
Q: Your research suggests a novel pathway for HCMV’s involvement in Alzheimer’s, involving transit from the gut to the brain via the vagus nerve. Could you explain the proposed mechanisms of this transport, including the role of viral latency and reactivation? What evidence supports the vagus nerve’s vulnerability?
A: We suspect that the vagus nerve, which connects the gastrointestinal tract to the brainstem, may act as a “conduit.” We observed HCMV in both the gut and brain of certain patients, and the vagus nerve’s extensive reach makes it a likely highway for viral travel. However, there may be alternative routes: HCMV can infect immune cells (monocytes, macrophages) that circulate in the blood and can potentially cross the blood-brain barrier.
Latency and reactivation could also be crucial. HCMV can remain dormant for long periods, reemerging under conditions of immune stress or compromise. We aim to use animal models to track precisely how the virus moves, including possible spread through infected immune cells or cranial nerves.
Q: Once HCMV reaches the brain, what mechanisms drive its contribution to Alzheimer’s pathology?
A: We’ve learned HCMV infects not only neurons but also microglia and other glial cells. In “mini-brain” organoids, HCMV infection dramatically increases the production of amyloid-beta and phosphorylated tau—the two proteins most often linked to Alzheimer’s disease – which may accelerate the formation of plaques and tangles, thus potentially amplifying neurological damage.
Moreover, HCMV infection of microglia can induce these cells to express CD83, matching what we see in human Alzheimer’s brains. This solidifies the idea that the virus is directly shaping the local immune environment in ways that promote neurodegeneration.
Q: How do you identify and distinguish this unique subtype of Alzheimer’s disease?
A: We ruled out common genetic and demographic factors, such as APOE status, age, and sex, which didn’t differentiate these patients. Our biggest clue came when we observed increased IgG4 in the gut of these patients, and later found that this was IgG4 against HCMV. This motivated our efforts to develop a blood-based biomarker strategy that focuses on the IgG4 antibody signature tied to HCMV so that we can begin to identify these patients in the clinic. Only a subset of the population appears to harbor chronic intestinal HCMV infection, suggesting certain immune profiles or genetic backgrounds might predispose individuals to reactivation in the gut.
It’s this persistent gut infection, plus the virus’s ability to travel to the brain, that underpins our classification of a distinct HCMV-related Alzheimer’s subtype.
Q: What can you tell us about the blood test being developed for detecting chronic HCMV infection?
A: We’re engineering a test that looks for specific antibodies in the blood—the IgG4 response—that we believe correlates strongly with both gut HCMV and CD83-positive microglia in the brain. Our target is at least 80% accuracy so we can reliably identify patients who might benefit from antiviral therapies or other specialized interventions.
Early results are promising, but we need more validation. If successful, this test could become a powerful tool for enrolling patients in clinical trials aimed determining whether antiviral therapies could halt or slow the progress of disease in these patients.
Q: Could this discovery of an HCMV-related Alzheimer’s subtype lead to more targeted treatment approaches?
A: Potentially, yes. While these patients still have the hallmark amyloid and tau pathologies, the viral component might demand additional or alternative therapies, such as antivirals or drugs that modulate the immune response. Trials will be needed to see whether combining existing anti-amyloid medications with treatments aimed at suppressing HCMV is more effective than amyloid-focused approaches alone.
Q: How does your research reshape our understanding of Alzheimer’s disease and its relationship with infections?
A: It underscores how the gut-brain axis can play a significant role in neurodegenerative diseases. We’ve shown that chronic gut infections don’t just stay in the gut; they can alter immune dynamics and influence brain health. Not everyone with HCMV gets this Alzheimer’s subtype, which makes sense—host factors and immune tolerance undoubtedly matter.
This model of a “common pathogen plus susceptible host” provides a framework for exploring other possible microbial contributions to neurodegeneration. It also shines a spotlight on the immune system’s delicate balancing act between fighting pathogens and avoiding harmful overreactions.
Q: Looking ahead, how do you see these findings shaping future Alzheimer’s research?
A: The immediate need is replication—we hope other groups will confirm that CD83-positive microglia, HCMV presence, and IgG4 elevations show up in additional populations. We’ve observed these findings in two cohorts so far (in Arizona and Chicago), but broader demographic testing is essential.
From a clinical standpoint, we’re eager to pursue antiviral or antimicrobial therapy trials in patients positive for chronic HCMV infection. Ultimately, this might enable a precision-medicine approach, tailoring treatments to each patient’s unique underlying disease mechanisms.
Q: How did collaboration between different institutions contribute to your research findings?
A: This was a team effort from multiple organizations:
Banner Alzheimer’s Institute & Brain and Body Donation Program (AZ): Provided the matched brain and body tissue samples—an extraordinary resource enabling us to pinpoint HCMV in both the gut and brain of the same individuals.
Arizona State University: Led the analysis of tissue samples and immunological studies, with Dr. Diego Mastroeni overseeing tissue profiling and histochemical studies.
UMass Medical School: Conducted the brain organoid experiments that showed HCMV infection rapidly boosting amyloid-beta and tau.
Icahn School of Medicine at Mount Sinai: Investigated microglia infection, replicating the cellular changes observed in actual human brain tissue.
Serimmune (CA): Performed the antibody analyses, revealing the central role of HCMV.
TGEN (Phoenix): Supplied single-nucleus RNA sequencing data that helped us identify CD83-positive microglia.
RUSH Alzheimer’s Disease Center: Shared brain tissue samples and data from the ROSMAP cohort so tha we could validate the association of CD83(+) microglia with HCMV.
Without each group’s specialized expertise, we couldn’t have pieced together the entire pathway from gut infection to brain pathology.
Q: How critical were Arizona’s biorepositories to your research?
A: Invaluable. The Brain and Body Donation Program at Banner Sun Health Research Institute doesn’t just store brain tissue—it maintains samples from multiple body sites for the same individual. This let us trace HCMV step by step from the gut to the spinal fluid to the vagus nerve and then to the brain, building a comprehensive picture of how a gut-resident virus might seed a neurodegenerative disease.
Maintaining such multi-tissue repositories is expensive and complex, but the insights gained are unparalleled. It’s a key reason we could connect the dots between this unique immune profile, HCMV, and Alzheimer’s pathology.
Q: What are your immediate research priorities moving forward?
A: Validation is our top priority—particularly for the blood-based biomarker. We must confirm that it reliably identifies the same HCMV-driven subtype in additional patient cohorts. Once validated, we can begin clinical trials to see whether interventions like antivirals or immune modulators can meaningfully change the disease trajectory in these patients.
Q: What key questions should other researchers explore to build upon your findings?
A: First and foremost, replication: does this HCMV association hold across multiple, diverse cohorts? Are there additional viral strains or different immune markers worth investigating?
We also need to clarify why some individuals develop chronic HCMV gut infections while others do not. Variations in genetic makeup, immune tolerance thresholds, or co-infections may all play a role. As we gather more data, we can refine our understanding of how significant this viral connection is in the broader Alzheimer’s population.
Q: What should the public understand about HCMV infection and its relationship to Alzheimer’s disease?
A: We all come into contact with HCMV and in most cases, it won’t cause any problems. Although HCMV is an important part of this story, the piece that seems relevant to disease is the chronic gut infection in a small subset of individuals—perhaps due to immune or genetic factors—as this seems to set the stage for HCMV to reach the brain and potentially contribute to Alzheimer’s pathology.
At this early stage, our study is best understood as a series of interesting scientific findings that warrant further exploration, replication, and validation in additional study populations. Although we are motivated to understand whether these findings have implications for clinical care, these studies have not been performed yet and so should not be used to guide clinical decisions.
Q: How could this research shape future public health strategies for Alzheimer’s disease?
A: If ongoing studies confirm that targeting HCMV can slow or prevent Alzheimer’s in susceptible patients, we may one day see screening and antiviral treatments introduced as a preventive measure. Though it’s not yet standard of care, the notion that infections and immune status play a key role in at least some forms of Alzheimer’s opens up new possibilities for intervention and prevention across the board.
Overall, this research is part of an emerging wave of precision medicine approaches, which recognize that Alzheimer’s isn’t one uniform disease. Identifying subtypes—like this HCMV-associated variant—could usher in more personalized therapies and better outcomes.
Conclusion
Published on 19 December 2024 in Alzheimer’s & Dementia, this pioneering study by Dr. Ben Readhead’s team offers fresh hope in the fight against Alzheimer’s by pinpointing a chronic gut infection that appears to spark disease processes in a significant fraction of patients. Though challenges remain—such as verifying the results in diverse populations and developing reliable antiviral strategies—these findings broaden our understanding of how viruses, the immune system, and the brain intersect.
If confirmed in larger trials, this breakthrough might lead to targeted treatments for an HCMV-related Alzheimer’s subtype, while shedding light on other ways that persistent infections contribute to neurodegeneration. Ultimately, for patients and caregivers who have long been frustrated by limited treatment options, these insights herald an era where antiviral and immune-focused therapies could combine with traditional Alzheimer’s drugs to deliver more effective—and more personalized—care.
xxxxx
Disclaimer:
The information provided in this article is intended for educational and informational purposes only and should not be construed as medical advice. The research discussed is based on preliminary findings and has not yet been widely validated. Always consult with a qualified healthcare professional before making any medical decisions or changes to your treatment plan. The authors and publishers are not responsible for any actions taken based on the information presented herein.