
Dr. Andrea Tedeschi’s Award-Winning Image Helps to Develop a New Treatment for Improving People’s Neurological Function
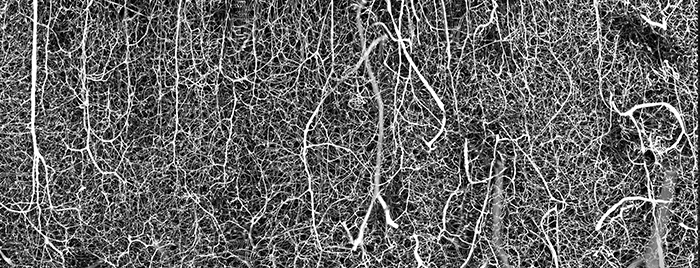
It is our great honor to be able to interview Dr. Andrea Tedeschi, Assistant Professor at the Ohio State University, Wexner Medical Center. His image of the 3D vasculature of an adult mouse brain was the winner of the 2021 Nikon Small World Photomicrography Competition. With Andrea’s interest in the complexity of the neural pathways in the mammalian central nervous system, this image better helps him to understand how changes in blood flow can impact the development of neurodegenerative diseases or prime functional recovery after stroke, brain, and spinal cord injury. Recently, Dr.Tedeschi discovered a new treatment for managing the usage of gabapentinoids, helping axon growth and regeneration after spinal cord injury in adult mice.

Q: Congratulations on your winning of the 2021 Nikon Small World Photomicrography Competition. Please share with us your background and interest in microscopic images.
A: The natural world has been my passion and inspiration since a young age. I studied violin at the Guido Cantelli Music Conservatory (Novara, Italy), biology and biotechnology at the Insubria University (Varese, Italy). My interest in microscopy developed over the course of the years.
As a neuroscience graduate student at the University of Tuebingen (Germany), I fell in love with the beauty of the nervous system. There, I spent countless hours imaging brain and spinal cord samples from transgenic mice expressing green fluorescent protein in a subset of neurons (PMID: 11086982). After obtaining my PhD degree in 2009, I did postdoctoral training at Boston Children’s Hospital – Harvard Medical School (Boston, US) and at the German Center for Neurodegenerative Diseases (DZNE) (Bonn, Germany). The experience in the laboratory of Dr. Frank Bradke at DZNE consolidated my interest in microscopy and 3D imaging technologies not as techniques but as powerful tools to dive deeper into the unknown. DZNE had a futuristic imaging facility equipped with standard and cutting-edge microscopes available to all members of the Institute. During that time, I learned how to use state-of-the-art multiphoton, light-sheet, and super-resolution microscopes. In December, 2016, I started my laboratory at the Ohio State University (OSU) – Wexner Medical Center where I have been surrounded by colleagues that share my passion for science. At OSU, I continued to have access to cutting-edge microscopy, tweak imaging protocols, and master sample preparation to gain a better signal-to-noise ratio, especially for 3D imaging applications.
Q: Please share with us the story behind your winning image of the 3D vasculature of an adult mouse brain and the techniques you used.
A: While imaging different classes of neurons across the central and peripheral nervous system under normal and pathophysiological conditions, I always asked myself “what about the vasculature?” As I started my laboratory at OSU, I started digging more deeply into the link between blood supply and brain function. To image the vasculature network down to the capillary level in the adult mouse brain, I perfected a method that was developed by Tsai, et al. (PMID: 19923289) and Di Giovanna, et al. (PMID: 30135559). Immediately after perfusing the mouse with a tissue fixative, I transcardially injected a fluorescent gel perfusate. I then dissected the brain, cleared it, and imaged it in 3D using a dedicated confocal microscope equipped with a long working distance objective. I cannot forget the first time I opened the image on the computer after spending a few hours scanning the mouse brain in 3D. Immediately after seeing the stunning image on the screen, I ran out of the microscope room looking for someone in the lab. Sharing the excitement of scientific discovery with the members of the laboratory is great!
Q: What have you learned from this winning image?
A: As a neuroscientist, I tend to focus on the neural pathways in the brain and central nervous system. Now that I have started digging more deeply into the link between blood supply and neuronal function, being able to see the complex details of the vasculature network in 3D is essential to better understand how changes in blood flow can impact the development of neurodegenerative diseases.

Q: You are a faculty in the Department of Neuroscience, Chronic Brain Injury Program at The Ohio State University – Wexner Medical Center. What are the main tasks of your lab and the goals it aims to achieve?
A: I am an Assistant Professor in Neuroscience at the Ohio State University – Wexner Medical Center. I am also affiliated with the Chronic Brain Injury Discovery Theme (https://discovery.osu.edu/cbi). My job is to provide guidance, advice, feedback, and support to all the members of the laboratory. I am deeply committed to high ethical standards, training and mentoring, scientific rigor and transparency. Our goal is to understand how to manipulate the self-repair mechanisms of the brain and spinal cord and help to design specific therapies aimed to improve neurological function and quality of life in individuals afflicted by central nervous system injury and neurodegenerative diseases.

Q: What is “in vivo time-lapse multiphoton microscopy”? How is it being utilized in your research?
A: Brain and spinal cord injuries cause profound neurological deficits and long-term disability due to detrimental alterations in the structure and function of neuronal circuits. Although neuronal injury is associated with several neurobehavioral and neuropathological characteristics, how changes in intrinsic neuronal properties alter the interaction between neurons and non-neuronal cells remains a central mystery in neuroscience. Much of the progress to address this question has come from either studies that use in vitro surrogate models or in vivo endpoint studies. These experimental models, however, do not provide the spectrum of variables that influence the behavioral or systemic changes that occur in response to injury or disease. By using in vivo time-lapse multiphoton microscopy, it is possible to image the system (both neuronal and vascular networks) ‘at play’ from hours to weeks and months after damage to the brain or spinal cord.
Q: If you don’t mind, please tell us some of the therapies that you have designed to improve people’s neurological function.
A: During development, embryonic neurons experience a drastic decline in axon growth capacity as axons approach their target field, thereby allowing a motile growth cone to differentiate into a presynaptic terminal specialized for neurotransmitter release. During my postdoctoral training, I questioned whether the transition from the growing to the transmitting phase represents one of the first key steps in the gradual loss of axon growth and regeneration ability. By using whole transcriptomic sequencing and bioinformatic analysis, followed by gain- and loss-of function experiments, I recently discovered that Cacna2d2, the gene encoding the Alpha2delta2 subunit of voltage-gated calcium channels, functions as a developmental switch that limits axon growth and regeneration. Cacna2d2 gene deletion or silencing promoted axon growth in vitro. In vivo, Alpha2delta2 pharmacological blockade through systemic Gabapentinoids administration promoted robust regeneration of sensory axons after spinal cord injury in adult mice. Gabapentinoids (e.g., pregabalin and gabapentin) are given to spinal cord injury individuals as a first-tier treatment to manage chronic neuropathic pain conditions. More recently, we found that Alpha2delta2 negatively regulates axon growth and regeneration of corticospinal neurons, the cells that originate the corticospinal tract. Increased Alpha2delta2 expression in corticospinal neurons contributed to loss of corticospinal regrowth ability during postnatal development and after SCI (Spinal cord injury). In contrast, Alpha2delta2 pharmacological blockade through gabapentinoids administration promoted corticospinal structural plasticity and regeneration in adulthood. We demonstrated that regenerating corticospinal axons functionally integrate into spinal circuits. Mice administered gabapentinoids recovered upper extremity function after cervical spinal cord injury. Importantly, such recovery relies on reorganization of the corticospinal pathway, as chemogenetic silencing of injured corticospinal neurons transiently abrogated recovery. We now have evidence that the same treatment strategy effectively promotes structural plasticity of corticospinal neurons and neurological recovery after stroke in mice. Interestingly, a multi-center, cohort study has found that SCI individuals receiving gabapentinoids have enhanced motor recovery if they are administered within the first months after injury. Together, these exciting results set up an opportunity to re-purpose Gabapentinoids as a novel treatment strategy to repair the injured central nervous system.
Q: What is your advice for aspiring students who are interested in neuroscience studies?
A: My advice for aspiring students interested in neuroscience studies is to be passionate and persistent.
With Dr. Tedeschi’s thorough and candid answers above, we learn how a readily available, clinically approved pharmacological treatment may be repurposed for people who suffer from stroke and spinal cord injury.