Dr. Yujia Zhang: Innovations in Bio-integrated Devices
The University of Oxford’s research team, under the leadership of Dr. Yujia Zhang, has achieved a remarkable breakthrough in the field of tiny bio-integrated devices capable of direct cellular stimulation. This pioneering work was recently highlighted in the esteemed Nature journal.
We had the privilege of interviewing Dr. Yujia Zhang. Here’s what he shared:
Q: Could you shed light on your academic background and the path that led you to lead this advanced research?
A: My scholarly journey began in China with an undergraduate degree in electronic engineering. I moved to Shanghai for my PhD in Bioengineering. Subsequently, I delved into biophysics research in New York, focusing on near-field nanospectroscopy. In 2021, I joined the Bayley group at the University of Oxford in the UK. While our team operates within the chemistry department, our research is truly cross-disciplinary, encompassing chemistry, biology, engineering, and materials. This multifaceted approach deeply aligns with my expertise, making our work fascinating.
Q: Your creation, the “droplet battery,” is being lauded as a groundbreaking innovation. Could you provide insights into this novel design?
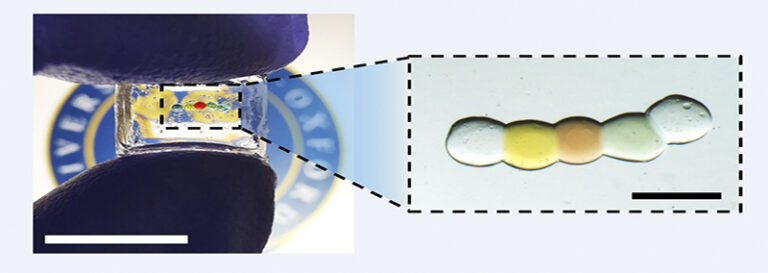
A: A decade ago, we initiated a droplet printing method, naming it “synthetic tissue.” This compact power source is derived from the layering of nanolitre-sized conductive hydrogel droplets. This hydrogel is basically a water-filled 3D polymer chain. Each droplet varies in composition, creating a salt gradient across the structure. Lipid bilayers provide stability and restrict ion movement between droplets, mirroring our cellular membranes. Compiling thousands of these droplets results in a network mimicking a droplet’s properties or what we define as synthetic tissue. In the years after this method’s inception, our discoveries were showcased in renowned journals like Science and, later, Nature. We aim to merge this synthetic tissue with real tissue, bridging the gap between man-made structures and genuine human organs. We theorized that our synthetic tissue might serve as a power source or a “battery” for further exploration. Our recent publication turned this theory into reality. Once encapsulated, the droplet battery can be used in wearables or even as an implant, integrating smoothly with internal human systems, whether organs, tissues, or neurons. Depending on its application, it can be internal or external, showcasing its true versatility.
Q: Could you delve into what inspired this design? What motivated you to take this unique research direction?
A: Indeed. Our environment is filled with marvels, with nature often serving as a rich source of inspiration for scientific advancements. We are proponents of biomimicry, where we mirror nature’s designs and processes in our technological pursuits. Our curiosity was piqued by animals like electric eels, capable of producing electricity. They have specialized cells, electrocytes, that can generate impressive ionic outputs, some even exceeding 600 volts in total. This natural wonder greatly influenced us. It prompted the question: Can we replicate this in a controlled, synthetic setting? Our subsequent endeavors led to the droplet network, designed to imitate the cellular structure that produces these ionic currents in the animal kingdom. We incorporated lipid bilayers, reflecting natural cellular barriers, to form the droplets chain. The charge-selective droplets between the high-salt and low-salt droplets mimic the membrane ion channels, which are instrumental in this process. In essence, our design pays homage to nature’s brilliance and showcases the potential of deriving inspiration from the natural world.
Q: I understand lipid bilayers play an integral role in this design. Are these bilayers a common feature in human biology?
A: Absolutely. Lipid bilayers are foundational structures in biology. They form the essential cell membranes that encapsulate the contents of human cells, as well as the cells of all other animals. These membranes serve as barriers and gatekeepers, allowing certain substances in while keeping others out. Our research, while inspired by this natural occurrence, does introduce a twist. While the lipid bilayers in our bodies are organic and formed from natural lipids, in our design we’ve employed synthetic lipids. Even though these are lab-created, they fundamentally mirror the properties and functions of their natural counterparts. In essence, our synthetic lipid bilayers act as scaffolds where we can place our specialized droplets, enabling us to form the droplet network constructs.
Q: Can you provide a comprehensive overview of the design and its intricacies?
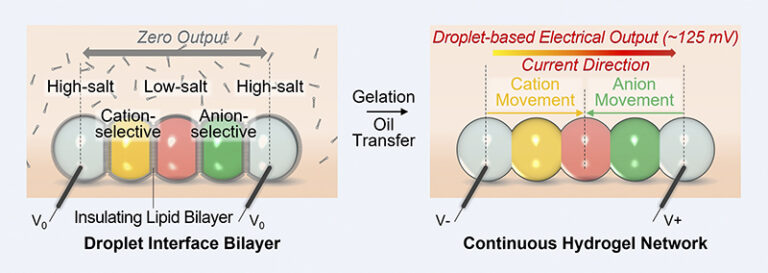
A: Absolutely. At the heart of our innovative design is a meticulously constructed droplet power source unit. This unit is composed of five individual droplets, each playing a pivotal role in generating power. On the outer extremities, we have droplets with a high salt concentration, which essentially serve as the power source’s “terminals.” Positioned centrally is a droplet with a markedly low salt concentration, even akin to pure water. These extremes of concentration, high and low, are crucial for establishing an ion gradient.
The interfacing droplets between the high salt and low salt ones are specialized: one permits only positive ions, while the other allows negative ions. This carefully arranged sequence, moving from left to right, goes as follows: high salt, cation-selective, low salt, anion-selective, and then another high salt droplet. Due to this design, positive ions (cations) migrate from the left-most droplet to the center, while anions journey from the right-most droplet to meet in the middle. This orchestrated movement establishes a current direction from left to right, constituting a single power unit.
The beauty of our design is its modularity. Leveraging droplet printing technology, we can seamlessly integrate tens or even hundreds of these units, akin to configuring batteries in series or parallel to amplify voltage or current. Such configurations are fully customizable, tailor-made to individual requirements. Our proprietary 3D printer, specifically engineered for this task, enables precise development of these droplet networks. Depending on specific human conditions or applications, diverse unit combinations can be crafted.
The power source unit isn’t merely a theoretical concept. It’s brought to life by depositing a chain of nanolitre-sized droplets of conductive hydrogel (a water-rich 3D polymer network). Each droplet varies in composition to form the desired salt gradient. Lipid bilayers play an instrumental role here, both separating droplets and providing essential mechanical support, while also inhibiting uncontrolled ion flow. Activation of this power source involves cooling it to 4°C and altering its surrounding medium, leading the lipid bilayers to disrupt and droplets to merge into a unified conductive hydrogel.
Consequently, ions navigate through this hydrogel, with the resultant ion gradients being converted into electric power when connected to electrodes. Our research showcases the impressive longevity and resilience of this design: after 36 hours of storage, the droplet power source still yielded a similar current after switching on, with a peak power output of around 65 nW for a 50 nanoliter unit.
Q: Considering the potential applications, which human conditions can benefit from this pioneering design?
A: Our research, as outlined in a paper published in Nature, delved into modulating specific neuronal structures. By merging our droplet power source with neural microtissues (organoids)—essentially conglomerates of hundreds of neurons emulating early human brain developmental stages—we discerned the influence of ionic currents on neuronal activity. This interaction induces calcium waves, offering potential to stimulate neuron growth. But that’s just the tip of the iceberg. Our ongoing research envisions a broader application spectrum, including heart-related stimulations. In real-world scenarios, our micro-scaled soft droplet units can be an alternative method to large-scale rigid electrical devices, harnessing the electricity to stimulate cells, tissues, and vital organs.
Q: Given that the power originates from the salt gradient within the ionic droplet, is it feasible to employ a different elemental source?
A: This is one of the key strengths and flexibilities inherent in our design. Currently, while we utilize an ion gradient as our primary energy source, the platform can easily accommodate alternative energy-generating materials and configurations, depending on the energy-generating mechanisms. Additionally, it’s worth noting that the hydrogel components used for constructing these droplets are biocompatible, ensuring safety in biological applications.
Q: What obstacles did you encounter in the development of this innovative device?
A: The development journey of our device was filled with unique challenges. One of the most pronounced hurdles was its potential application within living organisms or in vivo. While our initial successes were with isolated or in vitro experiments, transitioning to in vivo applications posed significant issues. The biological environment inside a living organism is complex. For instance, when introduced into this environment, the salts within our device could easily disperse into surrounding tissues and fluids. This dissipation poses a problem because our device’s functionality heavily relies on maintaining specific salt gradients.
To combat this, we encapsulated the system using an organogel. This approach provided a preliminary solution to contain and preserve the inner environment of the device. However, a lasting challenge remains: to develop a robust encapsulation mechanism that meets specific in vivo physiological conditions. This challenge is at the forefront of our ongoing research.
Q: How does transitioning from isolated droplets to a continuous hydrogel enhance the device’s functionality? And could you shed light on the role of the 4°C cooling step?
A: This transition is pivotal for our device’s operation. Initially, the lipid bilayers between the droplets act as barriers, preventing ions from migrating between droplets. These bilayers essentially place the device in an “off” state. The temperature decreases and droplets gelation disrupts these barriers, facilitating the droplets’ fusion into a continuous conductive hydrogel. This transformation effectively “turns on” our device, allowing ions to traverse the hydrogel, setting off the power generation process.
Q: Is there a mechanism to remotely control this activation process?
A: Yes, we’re continually exploring more user-friendly and versatile activation mechanisms. In our subsequent research, we’ve been investigating light as an alternative trigger. The idea is to leverage light’s capability to penetrate certain depths within tissues. By embedding or surrounding the droplets with light-sensitive components, we can achieve activation by merely shining light on the system. This approach not only provides remote control but is also non-invasive and straightforward, broadening potential applications.
Q: Can you provide insights into the device’s longevity, especially in practical scenarios?
A: Our findings have shown that once activated, our device consistently generates power for durations ranging from 30 minutes up to two hours. Considering its biodegradable nature, the envisioned application within living organisms would be transient. We see it as a one-time-use system, wherein post-activation, it delivers power for its operational lifespan and eventually degrades harmlessly. However, outside living organisms, the device can be recharged. By connecting electrodes and applying a reverse voltage, ions can be redirected to their original positions, effectively “resetting” the device.
Q: What potential therapeutic avenues does this technology open up?
A: Our primary exploration centered around neuronal modulation. Through our device, we’ve observed enhanced neuronal connectivity and maturation following prolonged culture. This finding holds promise for therapeutic applications in neurology sciences. Our ongoing research aims to expand these findings and uncover additional therapeutic opportunities, for example in cardiovascular treatments.
Q: Could you delve into the device’s modular design and how it allows for customization?
A: Absolutely. Our design’s modularity is one of its key strengths. By combining multiple units, the overall output can be amplified. Additionally, the salt gradient within each droplet can be adjusted during the fabrication process, enabling control over the device’s output. This flexibility ensures our device can be tailored to specific requirements, whether it’s adapting to different tissue types or adjusting to specific therapeutic needs. Given its soft and small-scale nature, our device can also be resized, making it compatible with various applications, from micro-scale interventions to more extensive treatments.
Q: With such promising outcomes, what are your plans for scaling production?
A: Our prototype was assembled using a customized 3D printer, allowing us to create up to 25 units in series. This modular approach lets us achieve higher voltages, making the device suitable for driving electronic circuits in future iterations. The primary goal, however, remains transitioning from in vitro to in vivo applications. Addressing the encapsulation challenge is vital before scaling production. Once that’s resolved, our platform can be adapted for numerous therapeutic and diagnostic purposes.
Q: Professor Bayley touched on the device’s potential in microbots and bio-hybrid interfaces. Can you expand on this vision?
A: Indeed, our device’s miniature and soft nature opens doors to pioneering applications. Imagine microbots navigated through blood vessels using external forces, like magnetism, carrying our device as an energy source. These microbots could be directed to specific locations within the body, delivering targeted therapies or interventions. Furthermore, the ionic basis of our device makes it an attractive candidate for bio-hybrid interfaces, potentially establishing seamless connections between biological systems and electronic devices.
Our multidisciplinary team, comprising chemists, biologists, and engineers, is working diligently to realize these futuristic visions. Their collective expertise has been indispensable in this journey, and I’m profoundly grateful for their contributions and the great support from the Bayley group and the Department of Chemistry, University of Oxford.