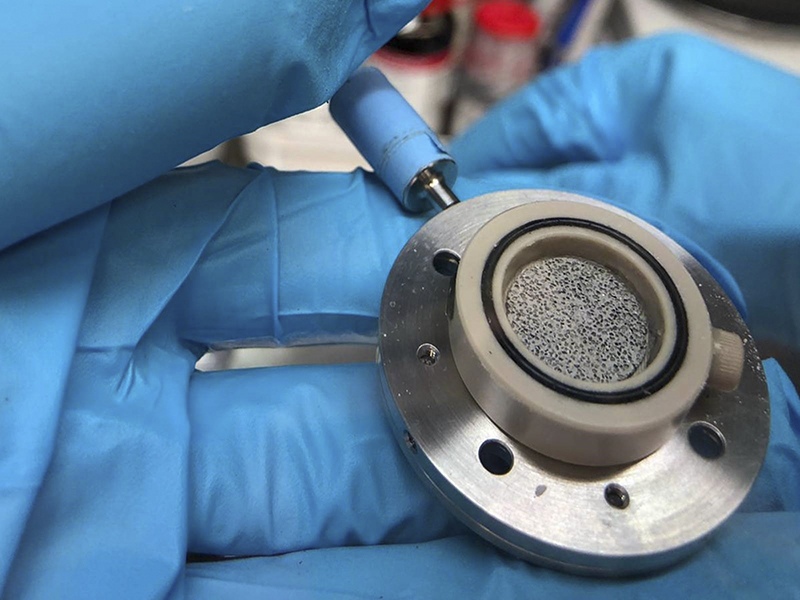
Scientists watch tin foam electrodes breathe under X-rays, solving battery expansion problems
Scientists have discovered that transforming tin into a highly porous foam structure may solve one of the biggest challenges facing next-generation batteries. This innovative approach, detailed in a recent study from Helmholtz-Zentrum Berlin (HZB), could pave the way for energy storage that packs significantly more power into the same space—think smartphones or electric vehicles with longer-lasting juice.
Beyond Graphite: The Metal Electrode Promise
For decades, lithium-ion batteries have leaned on graphite electrodes to shuttle lithium ions during charge and discharge cycles. Graphite’s reliable, but its capacity tops out at 372 mAh g⁻¹, pushing researchers to hunt for denser alternatives. Tin, with a theoretical capacity of 993 mAh g⁻¹—nearly triple graphite’s—stands out. “Tin’s abundant, non-toxic, and can store way more lithium ions,” says Dr. Sebastian Risse of HZB, co-author of the study published in Advanced Science. Trouble is, when lithium floods in, tin expands up to 260%, cracking and crumbling over time.
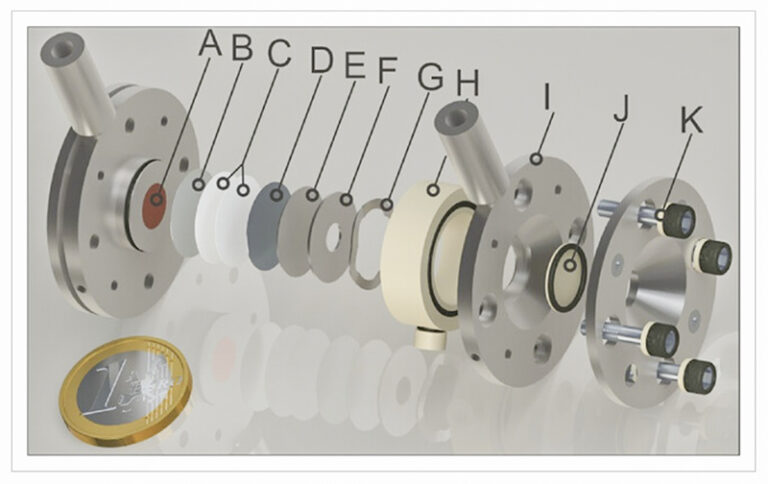
Watching Batteries Breathe
To crack this puzzle, the HZB team turned to operando X-ray imaging at BESSY II’s BAMline facility—a synchrotron marvel pumping out high-energy X-rays. “We watched tin electrodes live during cycling,” says lead author Dr. Bouchra Bouabadi. “It was like seeing the material breathe—expanding, contracting, fracturing.” Their high-resolution radioscopy, capturing images down to 0.83 μm/pixel, revealed lithium carving out “islands,” “peripheries,” and “floors” in solid tin foils. Cracks sprouted in the islands as lithium piled in, while floors held steady, hinting at uneven stress as the culprit behind failure.
The Foam Solution
Enter Dr. Francisco Garcia-Moreno’s brainchild: tin foam. This spongy structure, with 54% porosity from micrometer-sized pores, cushions the blow of expansion. “The pores give lithium room to settle, cutting mechanical stress,” explains Garcia-Moreno, a metal foam veteran. They crafted it via a powder metallurgy trick—mixing tin powder (< 44 μm, 99.8% pure) with ammonium hydrogen carbonate (200-320 μm), pressing at 300 MPa, then sintering under vacuum (140°C then 180°C for 2 hours each). The carbonate fizzed out, leaving a porous tin skeleton.
The foam’s debut at BESSY II, announced on February 24, 2025, via HZB’s newsroom, showcased its promise. Unlike solid foils, it flexed without shattering, a resilience tied to its tiny 22 μm grains—far smaller than the 260 μm grains in 100 μm solid foil. More grain boundaries meant more stress relief through slippage, a finding detailed in the Advanced Science paper.
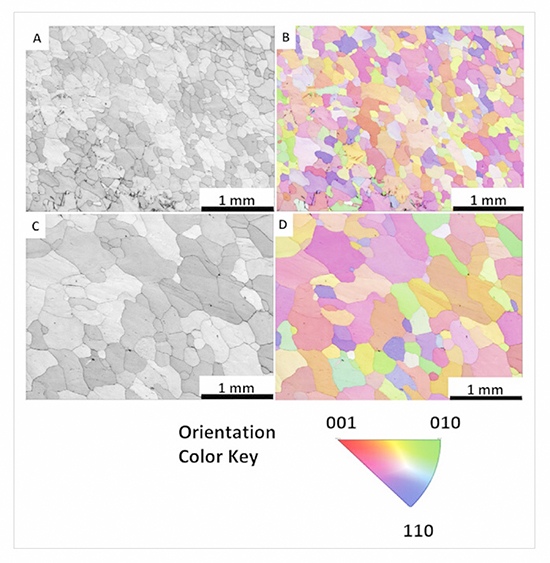
Breathing Behavior and Efficiency
The foam “breathed” in sub-steps—lithium filling pores in bursts, not a smooth sweep—offering a peek into porous electrode dynamics. Efficiency jumped too: solid foil started at a measly 33% Coulombic efficiency, while foam hit 48.25% in cycle one, climbing to 75.5% by cycle three. “It’s not just surviving—it’s reversing lithium loss better,” Risse notes. Solid tin flopped fast; foam kept going.
The Path Forward
Challenges linger: foam walls crack if too thick, and the solid electrolyte interphase (SEI) layer wobbles, sapping stability. “We’re eyeing thinner walls and tougher SEI recipes,” says Bouabadi. Cost-wise, tin foam beats fancy nanostructured electrodes while rivaling their perks, though it’s pricier than plain foil.
This HZB breakthrough, blending synchrotron wizardry with clever design, could jolt battery tech forward. With tin foam tackling expansion woes, next-gen storage—packing triple the punch of today’s batteries—edges closer for EVs and gadgets. As energy demands skyrocket, such innovations might just keep the world powered up.