
Revolutionizing Regenerative Medicine: Dr. Kristopher Kilian's Breakthrough with Trpzip Hydrogel
Researchers at the University of New South Wales (UNSW) Sydney have introduced a novel hydrogel called Trpzip, signifying a potential paradigm shift in regenerative medicine and tissue engineering. This development presents a fresh perspective on the methodologies of healing and tissue restoration. The findings have been documented in a recent issue of Nature Communications.
From Concept to Creation: Dr. Kilian's Journey with Trpzip Hydrogel
Our deep dive into this promising material began with an enlightening conversation with Dr. Kristopher Kilian, whose diverse educational and research experiences have crystallized into the creation of Trpzip hydrogel. From genomics to chemical biology, and material science to bioengineering, Dr. Kilian’s interdisciplinary approach has led to this momentous discovery that stands to benefit myriad medical applications.
The Discovery of Trpzip Hydrogel
The discovery, made during the COVID-19 lockdowns, was a result of computational simulations conducted by Ashley Nguyen, a PhD student at UNSW under Dr. Killan. Nguyen focused on molecules that self-assemble, leading to the discovery of a novel variant of the ‘tryptophan zipper’ (Trpzip), which are short chains of amino acids effective in promoting self-assembly. These zippers could form hydrogels just by stacking together, mirroring the structure and properties of natural materials without the need for harvesting animal tissu3, marking a substantial leap in material science and its applications in biomedical research.
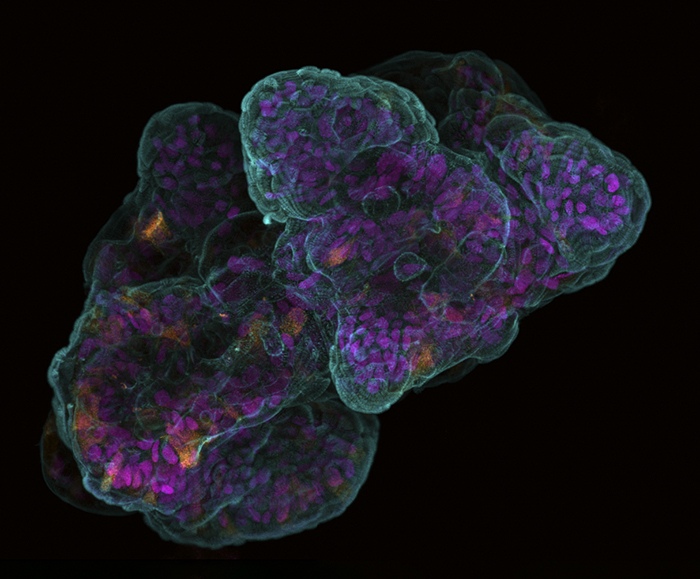
The Healing Gel: Trpzip's Unique Characteristics
Antimicrobial Action of Trpzip Hydrogel: Trpzip hydrogel’s antimicrobial prowess is particularly fascinating. This ability is largely attributed to its tryptophan-rich peptide sequences. Tryptophan, an essential amino acid, has been linked to antimicrobial activity in various studies. Ashley’s intuition about the potential antimicrobial nature of the hydrogel led to collaboration with Dr. Naresh Kumar, a specialist in antimicrobial agents. Through testing, the hydrogel demonstrated efficacy against both gram-positive and gram-negative bacteria. This broad-spectrum antibacterial action holds immense medical potential. While the precise molecular mechanisms remain to be fully understood, the practical implications are clear. In medical settings, Trpzip hydrogel could be revolutionary in preventing infections when used as a bandage or in post-operative care, thereby tackling the growing concern of antibiotic-resistant bacteria.
Mimicking Human Tissue: The Trpzip hydrogel’s ability to mimic human tissue is derived from its stress relaxation characteristics. Stress relaxation is vital because it describes the material’s ability to regain its shape after being deformed, a property inherent to many biological tissues like Matrigel and collagen. Unlike synthetic materials that may not return to their original state, Trpzip hydrogel closely emulates the natural tissue’s response to mechanical forces.
Furthermore, the hydrogel’s hierarchical ordering mimics natural tissue assembly, ranging from molecular to macroscopic scales. This structural hierarchy is pivotal because it leads to the gel’s distinctive mechanical properties, including its similarity to natural tissue with regard to stress relaxation behavior. Understanding and replicating this complex ordering is crucial for developing materials that not only support cell growth but also integrate seamlessly with the human body’s mechanical demands.
Self-Healing and Applications in 3D Bioprinting and Medical Treatments: The self-healing aspect of the Trpzip hydrogel is a cornerstone of its utility. Upon disruption, such as when passed through a syringe, the hydrogel’s peptides can reassemble into their original structure, a process that can occur within minutes to an hour. This rapid self-healing makes the hydrogel an excellent candidate for injectable therapies, where immediate gelation post-injection is crucial.
For 3D bioprinting, the hydrogel’s properties offer a substantial advantage. It can be extruded like a high-viscosity material, maintaining its shape upon deposition, and then solidify once in place. This behavior circumvents the need for cross-linking agents or additional curing steps, which are typically required to stabilize printed structures.
Furthermore, the self-healing feature enables the hydrogel to function as a supportive matrix for complex 3D printing applications. It can recover its integrity after the printing nozzle moves through it, which is essential when printing intricate structures within a gel matrix.
The potential to simplify post-printing processes is another remarkable aspect of the hydrogel’s self-healing ability. Instead of traditional methods that require laborious cleaning or enzymatic treatments to retrieve printed objects or grown tissues, the Trpzip hydrogel could be quickly and cleanly separated from the printed product through simple mechanical actions like shaking or sonication. This ease of material handling could streamline processes in tissue engineering and regenerative medicine.
In summary, the unique features of Trpzip hydrogel, from its antimicrobial properties to its bio-mimicry and self-healing abilities, offer significant advancements for medical applications. Its versatility and adaptability could revolutionize various aspects of healthcare, from surgical recovery to tissue engineering and beyond.
Addressing Global Challenges: The Road to Clinical Application
While the prospects of Trpzip hydrogel are undeniably promising, transforming this laboratory success into a clinically approved medical material is rife with challenges. Regulatory approval from bodies like the FDA is a significant hurdle, requiring extensive and costly animal testing to ensure safety and efficacy. The quest for funding this phase is an uphill battle, calling for strategic outreach to government agencies, philanthropic entities, and private foundations.
Equally daunting is the task of optimizing the hydrogel’s properties for varied medical applications. Its capacity to quickly dissolve can be problematic in situations where stability is key. Innovations like additives that respond to light to induce stiffness are under exploration to fine-tune the material’s behavior for specific uses.
Finally, the transition into the market demands overcoming the inherent conservatism within the medical industry. Convincing professionals to adopt an entirely new material, despite its advantages, requires a robust portfolio of evidence and the building of strategic alliances with industry thought leaders.
The Horizon of Healing: Future Perspectives on Trpzip Hydrogel
The journey of Trpzip hydrogel from a concept to a staple in medical treatments encapsulates the essence of scientific innovation: it’s arduous, laden with challenges, but profoundly rewarding. As the research progresses and moves closer to real-world applications, the promise held within this self-healing, bioactive, and antimicrobial substance could herald a new era of healthcare—where materials not only repair but regenerate, and medicine not only treats but truly heals.
In an age where the potential for material science to revolutionize medicine is vast, Trpzip hydrogel stands out as a beacon of hope and innovation—a testament to the power of interdisciplinary research and the relentless pursuit of scientific breakthroughs that can change lives.