
Smart Crystals Draw Inspiration from Desert Life to Revolutionize Water Harvesting
Water scarcity remains one of the world’s most pressing challenges, particularly in arid regions where access to clean water is increasingly limited. In a groundbreaking development, scientists have created an innovative solution inspired by nature’s own water-harvesting mechanisms, specifically those found in desert-dwelling creatures like beetles and lizards.
A collaborative research team from Jilin University, New York University Abu Dhabi’s Smart Materials Lab, and the NYUAD Center for Smart Engineering Materials has developed remarkable new crystalline materials called Janus crystals. These smart crystals can efficiently harvest water from fog without requiring any energy input, mimicking the natural adaptations of desert organisms that have evolved to survive in water-scarce environments.
Under the leadership of Professor Panče Naumov at New York University and New York University Abu Dhabi and Professor Hongyu Zhang from Jilin University, the team has successfully engineered these crystals to feature both hydrophilic and hydrophobic regions, creating a sophisticated water collection system that operates spontaneously under ambient conditions. This innovative approach represents a significant advancement over traditional, energy-intensive water procurement methods like desalination, offering a potentially sustainable solution for water collection in challenging, arid environments. The team presented their findings in a new paper published in the Journal of the American Chemical Society.
We recently had the opportunity to speak with Dr. Panče Naumov, Professor of Chemistry, to learn more about this remarkable research:
Q: What inspired the development of Janus crystals, and how did desert organisms influence your design?
A: Our project began with a fundamental goal: finding sustainable solutions for fresh water access. Our ongoing collaboration with Jilin University on crystalline materials naturally evolved into addressing this critical challenge, which is particularly relevant in the Middle East and worldwide.
The UAE currently relies heavily on desalination for about 96% of its potable water—an energy-intensive process that uses problematic membranes and fluorine-containing polymers categorized as “forever chemicals” that do not degrade after use.
Looking to move away from these unsustainable methods, we turned our attention to an untapped resource: atmospheric moisture. We realized that with the right materials, we could harvest water from humid air without the energy costs associated with desalination.
Nature provided our blueprint through the remarkable adaptations of desert organisms. The Namib Desert beetle, for example, harvests drinking water from fog using specialized surface structures that combine water-attracting and water-repelling areas. By studying these natural systems, we developed Janus crystals that mimic these biological water-harvesting mechanisms, creating an energy-efficient solution for water collection. This publication represents our success in translating nature’s time-tested strategies into engineered materials.
Q: Can you describe the specific organic compounds and structural components that make up the Janus crystals, and explain how each contributes to their hydrophilic and hydrophobic properties essential for efficient water harvesting?
A: The core idea behind the new concept of Janus crystals is to replicate the dual-surface strategy observed in certain desert organisms. These organisms rely on surfaces that have two distinct types of regions—one that readily attracts water (hydrophilic) and another that repels it (hydrophobic). In our synthetic approach, we needed to create a single crystalline material that effectively mimics this duality.
To achieve this, we started with organic crystals grown from three chemically diverse compounds. We chose organic materials because they are lightweight, non-polymeric, and potentially biodegradable, allowing for a sustainable approach. The key step was to treat only one side of the crystal with a hydrophobic coating via the process called silylation, leaving the other side unchanged and inherently hydrophilic. In essence, the crystal’s “Janus” nature—named after the Roman god with two faces—derives from having one side that strongly attracts and condenses water vapor and another that directs these condensed droplets along a hydrophobic surface toward a collection point.
By selecting different organic compounds and applying this half-and-half modification, we demonstrated that the concept works for a range of chemical structures. The result is a versatile, energy-free water-harvesting platform that takes advantage of natural phase transitions and dual-surface properties to efficiently capture and deliver atmospheric water.
Q: What crystal size do you believe is most efficient for water harvesting?
A: We’ve conducted extensive studies, examining factors like size and aspect ratio of the crystals, as well as the effects of various humidity levels and other conditions. Generally, we use crystals that are a few millimeters to a few centimeters in length. This size range makes them easy to handle and manipulate, which is important for both experimental work and potential practical applications.
Crystals in this size range also tend to have an elongated shape and a high surface-to-volume ratio. This configuration provides the key asset for efficient water collection—a large surface area available for water collection per unit mass and volume. By optimizing the composition, we’ve managed to produce crystals that are not only lightweight and easy to handle, but also highly effective at harvesting water.
Q: Could you explain how the hydrophilic and hydrophobic regions work together on your crystals, and what makes your approach “smart”?
A: The crystals have a unique design where the hydrophilic and hydrophobic regions are positioned on opposite sides of the elongated crystal structure. Naturally, the crystal surface is fairly hydrophilic, and we create the hydrophobic region by coating half of the crystal with a silicon-based coating—a process that could be as simple as dipping half the crystal in the polymer solution.
What makes our system particularly innovative is its self-sensing capability. These crystals are optically transductive, acting like optical waveguides that can guide light in their interior through total internal reflection. By shining light through one side of the crystal, we can monitor the water collection process in real-time. The light reflection or scattering at the surface of the crystals differ between crystal-air and crystal-water interfaces, allowing us to track when water droplets attach and detach from the surface.
We’ve tested this monitoring system under various conditions to ensure reliability. Looking ahead, we plan to create bundles of these crystals to increase the collection surface area, all monitored simultaneously. This will enable automated optimization of the water collection process through real-time feedback, making our crystals a truly smart, responsive technology.
Q: What is the life cycle of these crystals? Can they be used indefinitely?
A: In principle, these crystals can last indefinitely since they don’t dissolve in water. While we still need to conduct extended lifetime studies, their organic composition allows for continuous use. We’re also exploring biodegradable materials for certain applications—especially in remote locations—where the system could naturally decompose after a set period without leaving a harmful environmental footprint.
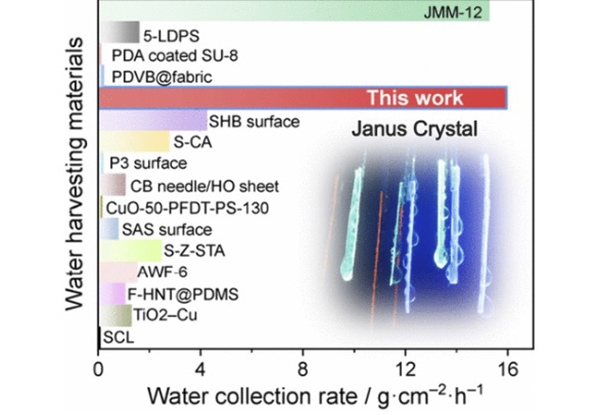
Q: How does the water collection efficiency of Janus crystals compare to existing atmospheric water harvesting methods?
A: Our crystals have proven to be record holders in terms of water collection efficiency, both per mass and per unit area. Comparative studies included in our publication show that only one material—a polymer net—comes close to our efficiency. However, our crystals work on a completely different principle and don’t contain non-degradable plastics, which is significant given the environmental concerns surrounding polymeric materials. The efficiency data, including collection times, is detailed in our paper.

Q: Do you foresee any challenges when scaling up this technology to an industrial level?
A: Transitioning from a laboratory concept to a fully industrialized product involves a period of optimization and engineering refinement. As chemists, we’ve provided the initial idea, but engineering considerations like mechanical stability, durability during transportation, resistance to vibration, and consistent real-world performance will need to be addressed by specialists. We hope that engineers and industry professionals will build on our work, refine the concept, and eventually develop a reliable, efficient system for large-scale use.
Q: Desalination is energy-intensive, whereas your method isn’t. Could you elaborate on the advantages of using Janus crystals over traditional desalination processes?
A: Traditional desalination reverses a naturally favorable process—the dissolving of salt in water—which requires significant energy. Whether it’s heating water for distillation or pushing it through membranes under pressure (reverse osmosis), there’s an inherent energy cost. Attempts to use solar power still don’t fully offset this.
Desalination also faces other challenges: membrane fouling, environmental footprint due to chemical cleaning, and the disposal of hyper-saline brine. In contrast, our Janus crystals rely on natural humidity fluctuations, requiring no external energy. By avoiding high-pressure systems, heating, and harmful chemicals, our method reduces environmental impact, energy consumption, and long-term ecological damage. Janus crystals represent a cleaner, more sustainable alternative to traditional desalination methods.
Q: Does the water collected by the crystal need additional purification before it can be consumed?
A: The condensation process naturally purifies the water by transforming it from a gaseous to a liquid state, leaving many impurities behind. This principle is similar to solvent purification by distillation and condensation in lab settings. Our goal is to enhance this natural process by capitalizing on a combination of surface properties, allowing for direct, low-energy access to relatively pure, potable water from the atmosphere without additional treatment.
Q: Could you describe the collaborative effort between the institutions involved in this research and its impact?
A: Our collaboration with Jilin University has been especially productive—over the past four to five years, we’ve published more than twenty papers together. This synergy, along with our international network, has helped establish organic crystals as a new class of materials with unique combinations of properties.
Our research’s significance is highlighted by recent funding from the Department of Defense, which is notable for a lab based in the Middle East. Such support underscores the global relevance and potential impact of our work.
Q: What are the temperature requirements and conditions for producing these crystals? Do you need high-temperature furnaces?
A: No, these crystals form at room temperature from organic solvents, removing the need for furnaces or high-temperature conditions. This simplicity sets them apart from many polymer-based materials, which are difficult to degrade and contribute to microplastic pollution. Our small-molecule organic crystals are environmentally benign and can potentially decompose naturally. This approach is particularly appealing now, as we recognize the downsides of polymers and seek alternatives that are both sustainable and practical.
Q: How can Janus crystals be integrated into water collection systems, and what optimizations are possible?
A: Scaling up can be as straightforward as using more crystals. We currently work with three different materials, allowing us to compare and fine-tune their hydrophobic and hydrophilic properties for optimal water absorption. Bundling the crystals together is a simple method to increase the water collection surface, but more complex water-collection device geometries could be developed to maximize exposure to humidity and efficiency. There’s ample room for innovation, both in terms of materials and design, to enhance overall performance.
Q: What potential impact could this technology have on water-scarce communities, and how do you envision its role in future water collection?
A: We need a paradigm shift in how we obtain fresh water. The atmosphere contains vast amounts of water—around 18,000 cubic kilometers—waiting to be tapped. While our approach may not match the efficiency of desalination in certain contexts, it shines in remote or mountainous regions where desalination isn’t practical.
Our technology serves as a middle-ground option, offering a sustainable method that isn’t as resource-intensive as desalination yet still provides reliable water collection. By harnessing nature’s principles, we’re adding a valuable tool to the spectrum of solutions for addressing global water scarcity.
Conclusion:
Janus crystals epitomize the convergence of nature-inspired design and cutting-edge materials science. By embracing strategies evolved in desert organisms, the collaborating research teams have crafted an energy-free, self-sensing platform that offers a sustainable alternative to conventional water-harvesting methods. While challenges lie ahead in scaling up, refining durability, and integrating these crystals into complex systems, the foundational achievements are clear. Janus crystals not only provide a pathway to cleaner, more efficient water collection but also highlight the transformative potential of biomimetic engineering.

